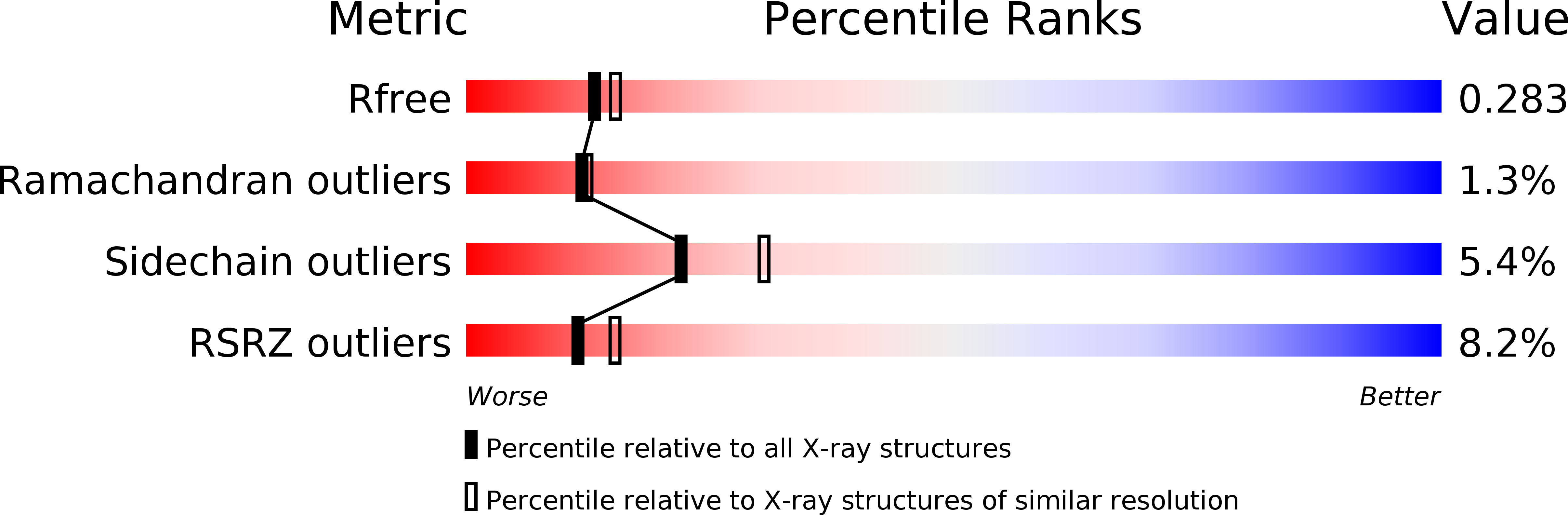A new modulated crystal structure of the ANS complex of the St John's wort Hyp-1 protein with 36 protein molecules in the asymmetric unit of the supercell.
Smietanska, J., Sliwiak, J., Gilski, M., Dauter, Z., Strzalka, R., Wolny, J., Jaskolski, M.(2020) Acta Crystallogr D Struct Biol 76: 653-667
- PubMed: 32627738
- DOI: https://doi.org/10.1107/S2059798320006841
- Primary Citation of Related Structures:
6SJJ - PubMed Abstract:
Superstructure modulation, with violation of the strict short-range periodic order of consecutive crystal unit cells, is well known in small-molecule crystallography but is rarely reported for macromolecular crystals. To date, one modulated macromolecular crystal structure has been successfully determined and refined for a pathogenesis-related class 10 protein from Hypericum perforatum (Hyp-1) crystallized as a complex with 8-anilinonaphthalene-1-sulfonate (ANS) [Sliwiak et al. (2015), Acta Cryst. D71, 829-843]. The commensurate modulation in that case was interpreted in a supercell with sevenfold expansion along c. When crystallized in the additional presence of melatonin, the Hyp-1-ANS complex formed crystals with a different pattern of structure modulation, in which the supercell shows a ninefold expansion of c, manifested in the diffraction pattern by a wave of reflection-intensity modulation with crests at l = 9n and l = 9n ± 4. Despite complicated tetartohedral twinning, the structure has been successfully determined and refined to 2.3 Å resolution using a description in a ninefold-expanded supercell, with 36 independent Hyp-1 chains and 156 ANS ligands populating the three internal (95 ligands) and five interstitial (61 ligands) binding sites. The commensurate superstructures and ligand-binding sites of the two crystal structures are compared, with a discussion of the effect of melatonin on the co-crystallization process.
Organizational Affiliation:
Faculty of Physics and Applied Computer Science, AGH University of Science and Technology, Krakow, Poland.