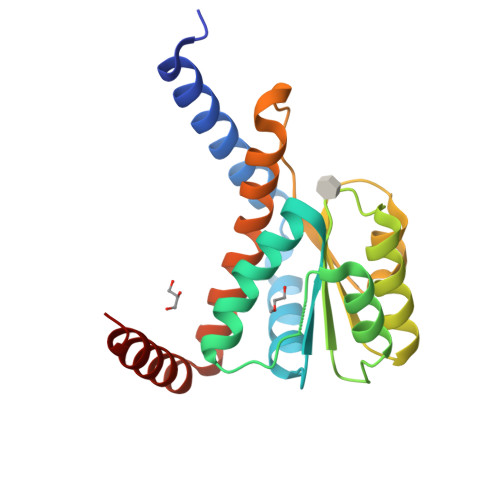
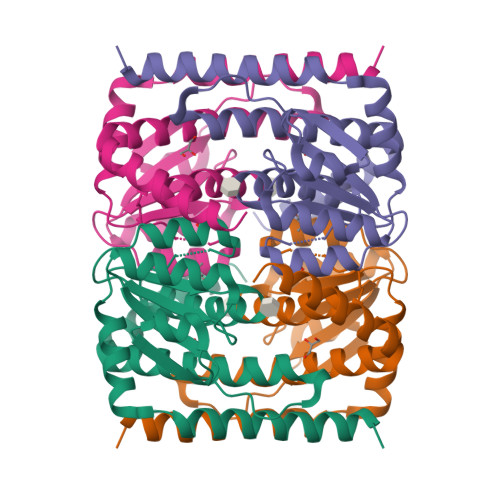
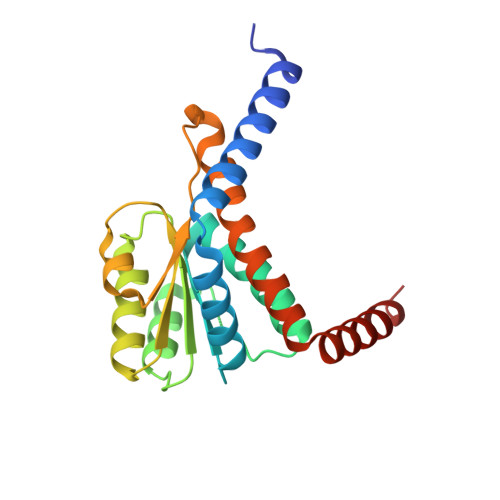
Molecular basis for cell-wall recycling regulation by transcriptional repressor MurR in Escherichia coli.
Zhang, Y., Chen, W., Wu, D., Liu, Y., Wu, Z., Li, J., Zhang, S.Y., Ji, Q.(2022) Nucleic Acids Res 50: 5948-5960
- PubMed: 35640608
- DOI: https://doi.org/10.1093/nar/gkac442
- Primary Citation of Related Structures:
7EN5, 7EN6, 7EN7 - PubMed Abstract:
The cell-wall recycling process is important for bacterial survival in nutrient-limited conditions and, in certain cases, is directly involved in antibiotic resistance. In the sophisticated cell-wall recycling process in Escherichia coli, the transcriptional repressor MurR controls the expression of murP and murQ, which are involved in transporting and metabolizing N-acetylmuramic acid (MurNAc), generating N-acetylmuramic acid-6-phosphate (MurNAc-6-P) and N-acetylglucosamine-6-phosphate (GlcNAc-6-P). Here, we report that both MurNAc-6-P and GlcNAc-6-P can bind to MurR and weaken the DNA binding ability of MurR. Structural characterizations of MurR in complex with MurNAc-6-P or GlcNAc-6-P as well as in the apo form revealed the detailed ligand recognition chemistries. Further studies showed that only MurNAc-6-P, but not GlcNAc-6-P, is capable of derepressing the expression of murQP controlled by MurR in cells and clarified the substrate specificity through the identification of key residues responsible for ligand binding in the complex structures. In summary, this study deciphered the molecular mechanism of the cell wall recycling process regulated by MurR in E. coli.
Organizational Affiliation:
School of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China.