Optimization of Benzothiazole and Thiazole Hydrazones as Inhibitors of Schistosome BCL-2.
Nguyen, W., Lee, E.F., Evangelista, M., Lee, M., Harris, T.J., Colman, P.M., Smith, N.A., Williams, L.B., Jarman, K.E., Lowes, K.N., Haeberli, C., Keiser, J., Smith, B.J., Fairlie, W.D., Sleebs, B.E.(2021) ACS Infect Dis 7: 1143-1163
- PubMed: 33523649
- DOI: https://doi.org/10.1021/acsinfecdis.0c00700
- Primary Citation of Related Structures:
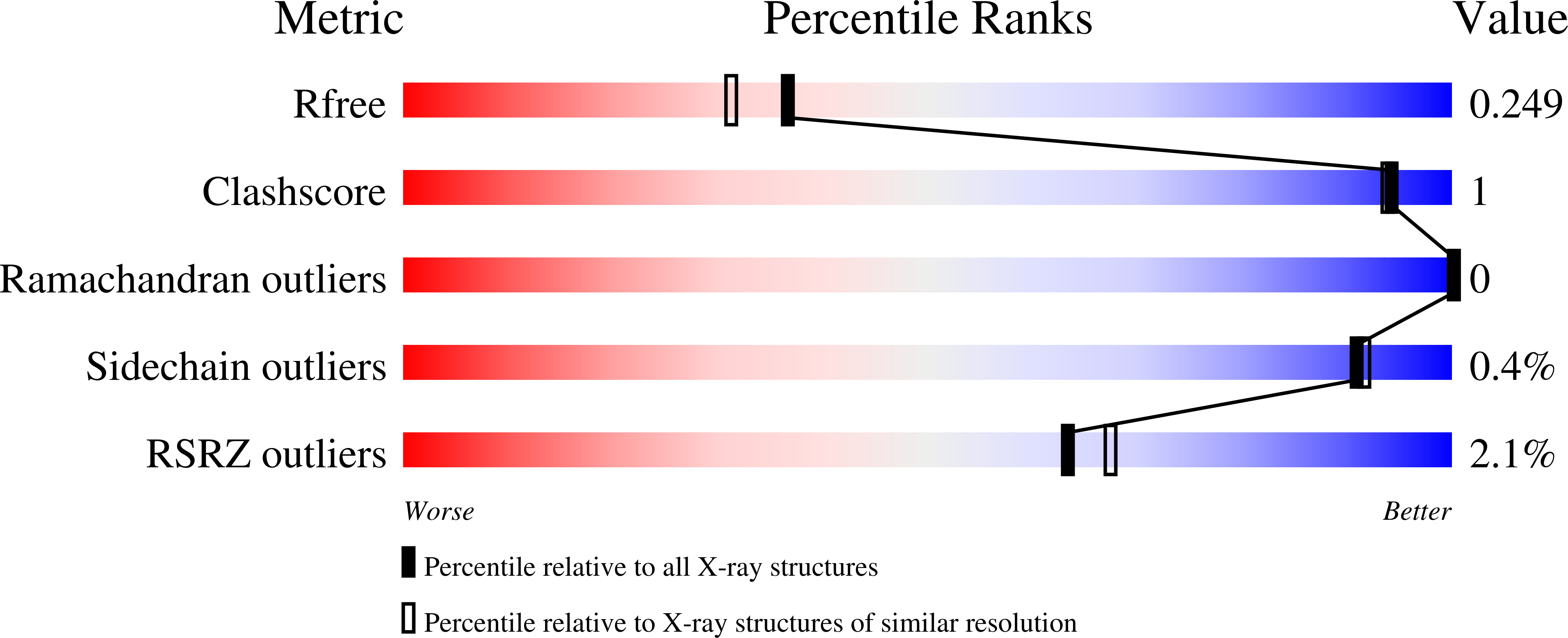
7JGV, 7JGW, 7JMT - PubMed Abstract:
Limited therapeutic options are available for the treatment of human schistosomiasis caused by the parasitic Schistosoma flatworm. The B cell lymphoma-2 (BCL-2)-regulated apoptotic cell death pathway in schistosomes was recently characterized and shown to share similarities with the intrinsic apoptosis pathway in humans. Here, we exploit structural differences in the human and schistosome BCL-2 (sBCL-2) pro-survival proteins toward a novel treatment strategy for schistosomiasis. The benzothiazole hydrazone scaffold previously employed to target human BCL-XL was repurposed as a starting point to target sBCL-2. We utilized X-ray structural data to inform optimization and then applied a scaffold-hop strategy to identify the 5-carboxamide thiazole hydrazone scaffold ( 43 ) with potent sBCL-2 activity (IC 50 30 nM). Human BCL-XL potency (IC 50 13 nM) was inadvertently preserved during the optimization process. The lead analogues from this study exhibit on-target activity in model fibroblast cell lines dependent on either sBCL-2 or human BCL-XL for survival. Further optimization of the thiazole hydrazone class is required to exhibit activity in schistosomes and enhance the potential of this strategy for treating schistosomiasis.
Organizational Affiliation:
The Walter and Eliza Hall Institute of Medical Research, Parkville 3052, Australia.