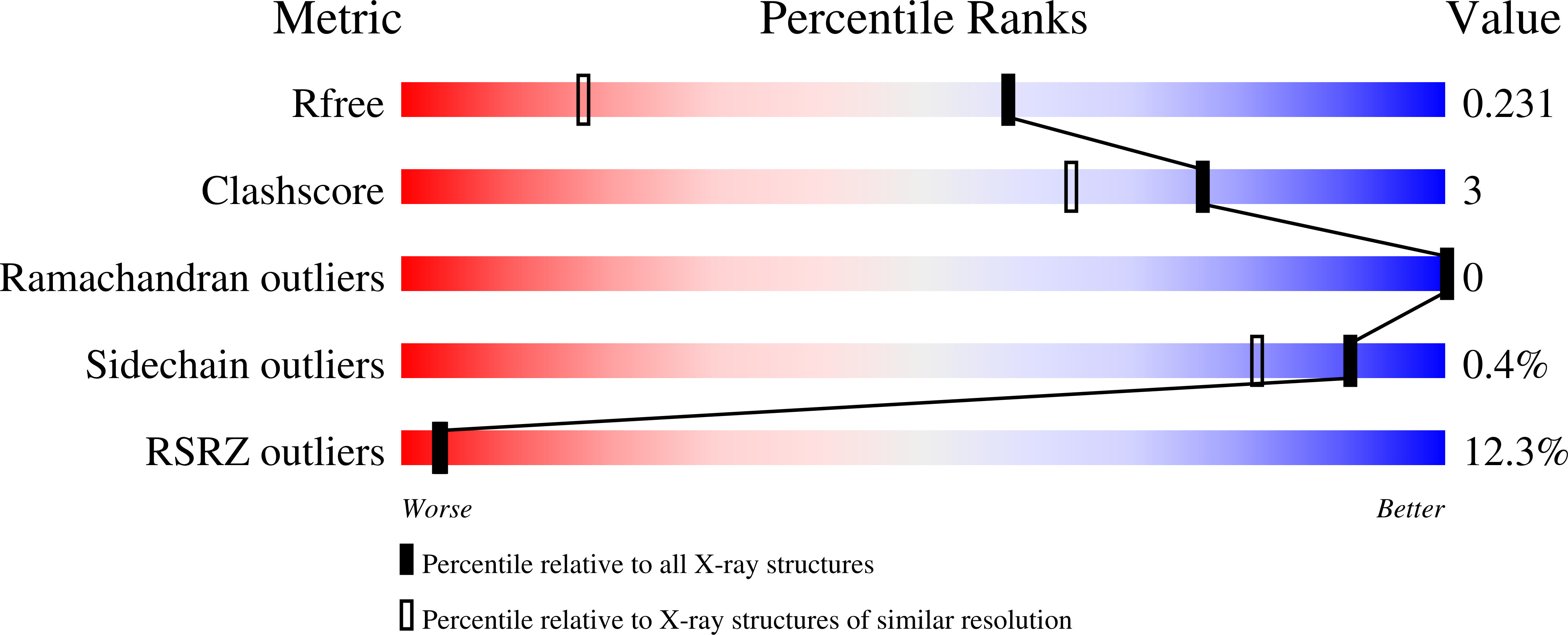
High-resolution structures of the bound effectors avadomide (CC-122) and iberdomide (CC-220) highlight advantages and limitations of the MsCI4 soaking system.
Heim, C., Hartmann, M.D.(2022) Acta Crystallogr D Struct Biol 78: 290-298
- PubMed: 35234143
- DOI: https://doi.org/10.1107/S2059798322000092
- Primary Citation of Related Structures:
7PS9, 7PSO - PubMed Abstract:
Cereblon (CRBN) is the substrate receptor of the CRL4 CRBN E3 ubiquitin ligase and is a central player in targeted protein degradation. It is the target of the thalidomide-derived immunomodulatory drugs (IMiDs) and is one of the most widely employed receptors for proteolysis-targeting chimeras (PROTACs), both of which induce the ubiquitination and subsequent proteasomal degradation of target proteins. Structural studies of ligand binding to CRBN are crucial to elucidate the mechanisms of action and for mediation of side effects, ultimately aiding the development of next-generation IMiDs and PROTACs. With this aim, a crystal-soaking system based on the single-domain bacterial homologue MsCI4 has previously been established and used to delineate the binding modes of several classes of small molecules, including FDA-approved drugs, at the molecular level. Here, this system was used to characterize the binding of the next-generation IMiDs avadomide (CC-122) and iberdomide (CC-220) at high resolution, highlighting the advantages and limitations of the MsCI4 system and its implications for the development of future cereblon effectors.
Organizational Affiliation:
Max Planck Institute for Developmental Biology, Max-Planck-Ring 5, 72076 Tübingen, Germany.