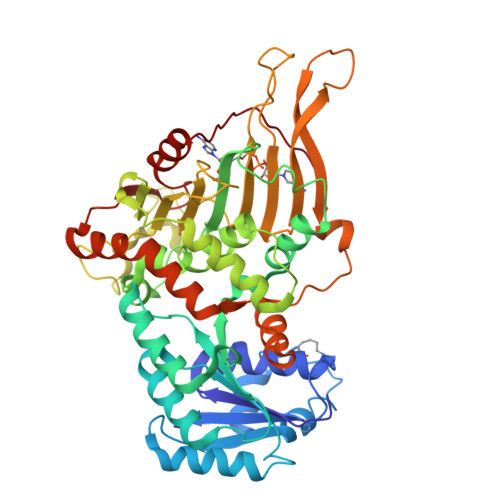
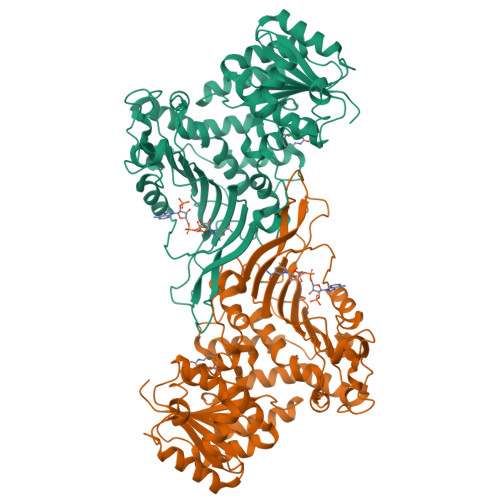
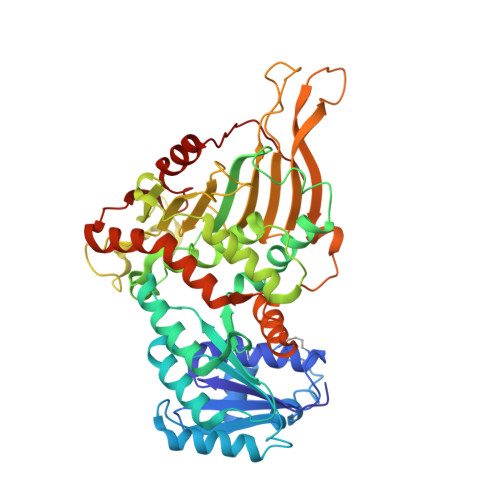
Acetylation-dependent coupling between G6PD activity and apoptotic signaling.
Wu, F., Muskat, N.H., Dvilansky, I., Koren, O., Shahar, A., Gazit, R., Elia, N., Arbely, E.(2023) Nat Commun 14: 6208-6208
- PubMed: 37798264
- DOI: https://doi.org/10.1038/s41467-023-41895-2
- Primary Citation of Related Structures:
7ZVD, 7ZVE - PubMed Abstract:
Lysine acetylation has been discovered in thousands of non-histone human proteins, including most metabolic enzymes. Deciphering the functions of acetylation is key to understanding how metabolic cues mediate metabolic enzyme regulation and cellular signaling. Glucose-6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme in the pentose phosphate pathway, is acetylated on multiple lysine residues. Using site-specifically acetylated G6PD, we show that acetylation can activate (AcK89) and inhibit (AcK403) G6PD. Acetylation-dependent inactivation is explained by structural studies showing distortion of the dimeric structure and active site of G6PD. We provide evidence for acetylation-dependent K95/97 ubiquitylation of G6PD and Y503 phosphorylation, as well as interaction with p53 and induction of early apoptotic events. Notably, we found that the acetylation of a single lysine residue coordinates diverse acetylation-dependent processes. Our data provide an example of the complex roles of acetylation as a posttranslational modification that orchestrates the regulation of enzymatic activity, posttranslational modifications, and apoptotic signaling.
Organizational Affiliation:
Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, 8410501, Israel.