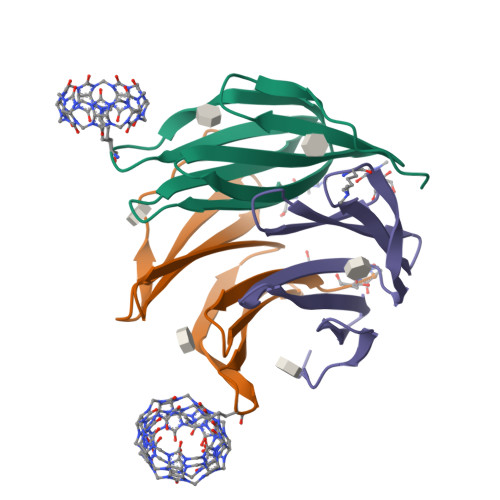
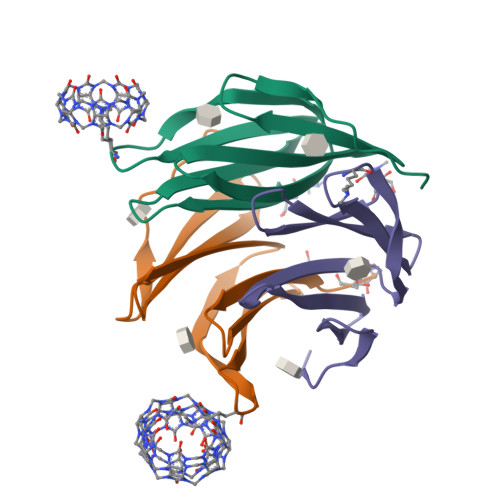
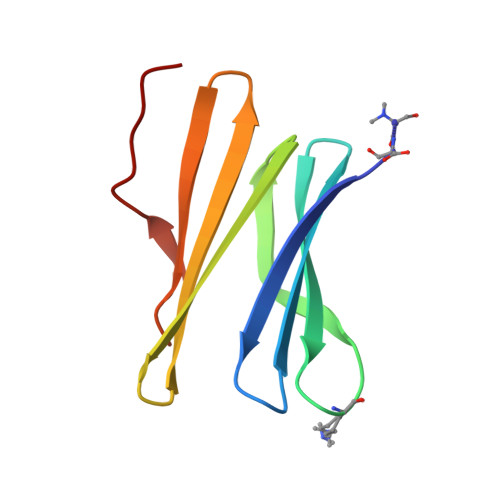
Cage versus sheet: Probing the Determinants of Protein - Cucurbit[7]uril Crystalline Architectures.
Ramberg, K.O., Crowley, P.B.(2023) J Struct Biol 215: 107969-107969
- PubMed: 37137399
- DOI: https://doi.org/10.1016/j.jsb.2023.107969
- Primary Citation of Related Structures:
8CF6, 8CF7 - PubMed Abstract:
The donut-shaped cucurbit[n]urils (Qn) are a class of rigid macrocyclic receptor with protein recognition capabilities. Qn encapsulation of amino acid side chains can enable protein assembly. Recently, cucurbit[7]uril (Q7) has been applied as a molecular glue for organizing protein building blocks into crystalline architectures. Q7 co-crystallization with dimethylated Ralstonia solanacearum lectin (RSL*) has yielded novel crystalline architectures. Co-crystallization of RSL* and Q7 yields either cage- or sheet-like architectures which may be modulated via protein engineering. However, questions remain as to the factors dictating the formation of one architecture over another (cage versus sheet). Here, we make use of an engineered RSL*-Q7 system which co-crystallizes as the cage or sheet assembly with easily-distinguished crystal morphologies. Using this model system, we probe how the crystallization conditions dictate which crystalline architecture is adopted. Protein-ligand ratios and the sodium concentration were identified as key determinants for the growth of the cage versus sheet assemblies.
Organizational Affiliation:
School of Biological and Chemical Sciences, University of Galway, University Road, Galway H91 TK33, Ireland. Electronic address: rambergk@tcd.ie.