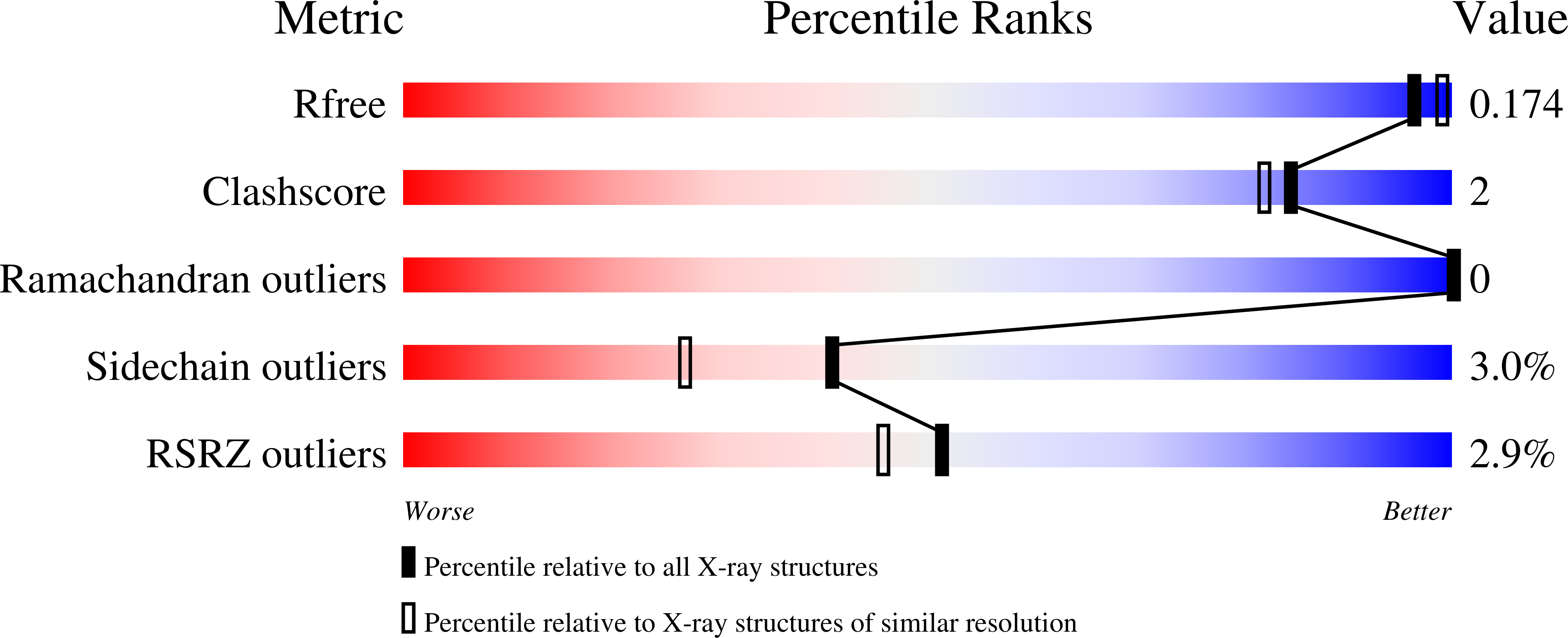
Obtaining anomalous and ensemble information from protein crystals from 220 K up to physiological temperatures.
Doukov, T., Herschlag, D., Yabukarski, F.(2023) Acta Crystallogr D Struct Biol 79: 212-223
- PubMed: 36876431
- DOI: https://doi.org/10.1107/S205979832300089X
- Primary Citation of Related Structures:
8EZO, 8EZP, 8EZU, 8EZX, 8F00, 8F01, 8F03, 8F05, 8F06, 8F07, 8F0B - PubMed Abstract:
X-ray crystallography has been invaluable in delivering structural information about proteins. Previously, an approach has been developed that allows high-quality X-ray diffraction data to be obtained from protein crystals at and above room temperature. Here, this previous work is built on and extended by showing that high-quality anomalous signal can be obtained from single protein crystals using diffraction data collected at 220 K up to physiological temperatures. The anomalous signal can be used to directly determine the structure of a protein, i.e. to phase the data, as is routinely performed under cryoconditions. This ability is demonstrated by obtaining diffraction data from model lysozyme, thaumatin and proteinase K crystals, the anomalous signal from which allowed their structures to be solved experimentally at 7.1 keV X-ray energy and at room temperature with relatively low data redundancy. It is also demonstrated that the anomalous signal from diffraction data obtained at 310 K (37°C) can be used to solve the structure of proteinase K and to identify ordered ions. The method provides useful anomalous signal at temperatures down to 220 K, resulting in an extended crystal lifetime and increased data redundancy. Finally, we show that useful anomalous signal can be obtained at room temperature using X-rays of 12 keV energy as typically used for routine data collection, allowing this type of experiment to be carried out at widely accessible synchrotron beamline energies and enabling the simultaneous extraction of high-resolution data and anomalous signal. With the recent emphasis on obtaining conformational ensemble information for proteins, the high resolution of the data allows such ensembles to be built, while the anomalous signal allows the structure to be experimentally solved, ions to be identified, and water molecules and ions to be differentiated. Because bound metal-, phosphorus- and sulfur-containing ions all have anomalous signal, obtaining anomalous signal across temperatures and up to physiological temperatures will provide a more complete description of protein conformational ensembles, function and energetics.
Organizational Affiliation:
SMB, Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, 2575 Sand Hill Road, Menlo Park, CA 94025, USA.