Discovery of the First Subnanomolar PPAR alpha / delta Dual Agonist for the Treatment of Cholestatic Liver Diseases.
Feng, Z., Xiang, J., Sun, G., Liu, H., Wang, Y., Liu, X., Feng, J., Xu, Q., Wen, X., Yuan, H., Sun, H., Dai, L.(2023) J Med Chem 66: 7331-7354
- PubMed: 37243609
- DOI: https://doi.org/10.1021/acs.jmedchem.2c02123
- Primary Citation of Related Structures:
8HF8 - PubMed Abstract:
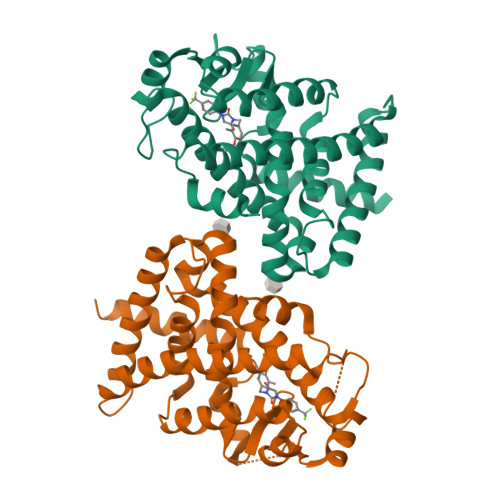
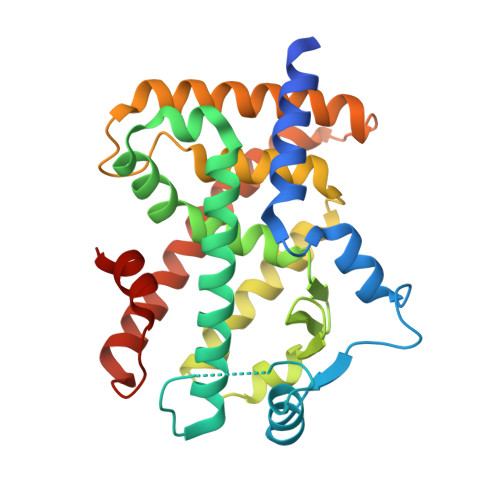
Peroxisome proliferator-activator receptors α/δ (PPARα/δ) are considered as potential drug targets for cholestatic liver diseases (CLD) via ameliorating hepatic cholestasis, inflammation, and fibrosis. In this work, we developed a series of hydantoin derivatives as potent PPARα/δ dual agonists. Representative compound V1 exhibited PPARα/δ dual agonistic activity at the subnanomolar level (PPARα EC 50 = 0.7 nM; PPARδ EC 50 = 0.4 nM) and showed excellent selectivity over other related nuclear receptors. The crystal structure revealed the binding mode of V1 and PPARδ at 2.1 Å resolution. Importantly, V1 demonstrated excellent pharmacokinetic (PK) properties and a good safety profile. Notably, V1 showed potent anti-CLD and antifibrotic effects in preclinical models at very low doses (0.03 and 0.1 mg/kg). Collectively, this work provides a promising drug candidate for treating CLD and other hepatic fibrosis diseases.
Organizational Affiliation:
Jiangsu Key Laboratory of Drug Discovery for Metabolic Disease, State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing 210009, China.