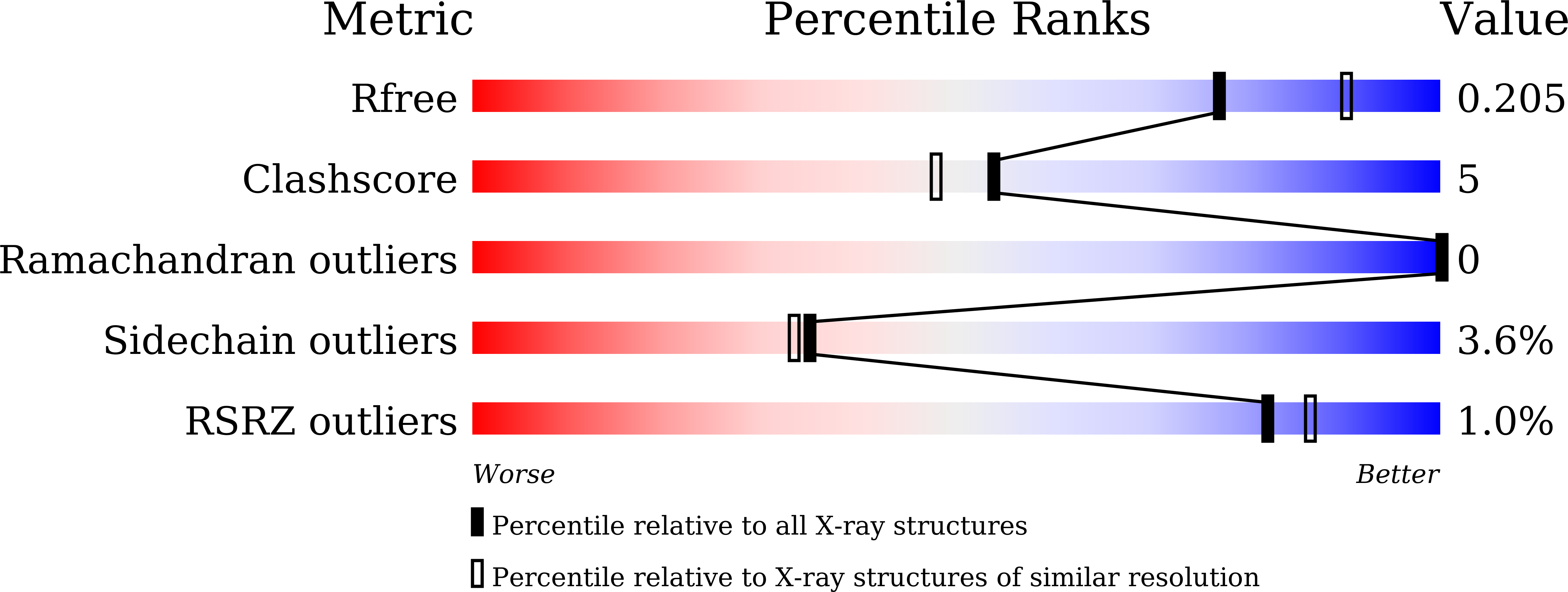
Characterization and alteration of product specificity of Beijerinckia indica subsp. indica beta-fructosyltransferase.
Li, D., Miyasaka, Y., Kubota, A., Kozono, T., Kitano, Y., Sasaki, N., Fujii, T., Tochio, T., Kadota, Y., Nishikawa, A., Tonozuka, T.(2023) Biosci Biotechnol Biochem 87: 981-990
- PubMed: 37280168
- DOI: https://doi.org/10.1093/bbb/zbad074
- Primary Citation of Related Structures:
8I2Q, 8I2R - PubMed Abstract:
The trisaccharide 1-kestose, a major constituent of fructooligosaccharide, has strong prebiotic effects. We used high-performance liquid chromatography and 1H nuclear magnetic resonance spectroscopy to show that BiBftA, a β-fructosyltransferase belonging to glycoside hydrolase family 68, from Beijerinckia indica subsp. indica catalyzes transfructosylation of sucrose to produce mostly 1-kestose and levan polysaccharides. We substituted His395 and Phe473 in BiBftA with Arg and Tyr, respectively, and analyzed the reactions of the mutant enzymes with 180 g/L sucrose. The ratio of the molar concentrations of glucose and 1-kestose in the reaction mixture with wild-type BiBftA was 100:8.1, whereas that in the reaction mixture with the variant H395R/F473Y was 100:45.5, indicating that H395R/F473Y predominantly accumulated 1-kestose from sucrose. The X-ray crystal structure of H395R/F473Y suggests that its catalytic pocket is unfavorable for binding of sucrose while favorable for transfructosylation.
Organizational Affiliation:
Department of Applied Biological Science, Tokyo University of Agriculture and Technology, Fuchu, Tokyo, Japan.