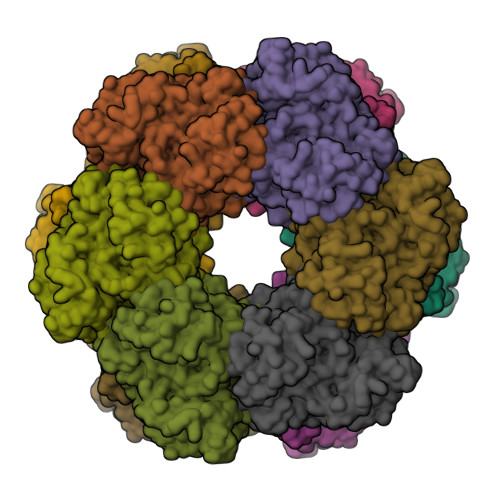
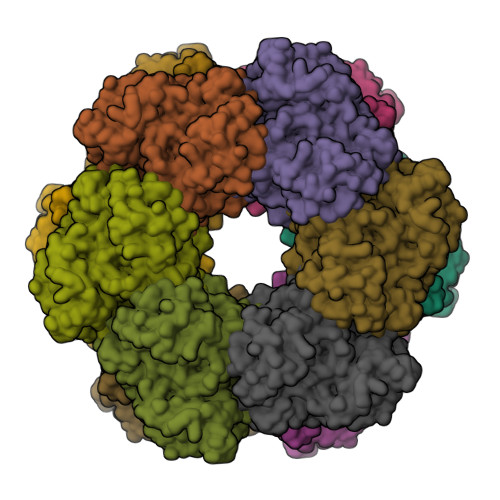
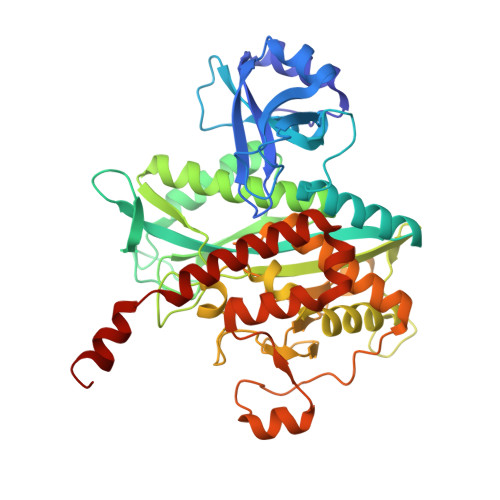
2-oxoglutarate triggers assembly of active dodecameric Methanosarcina mazei glutamine synthetase.
Herdering, E., Reif-Trauttmansdorff, T., Kumar, A., Habenicht, T., Hochberg, G., Bohn, S., Schuller, J., Schmitz, R.A.(2025) Elife 13
- PubMed: 40163028
- DOI: https://doi.org/10.7554/eLife.97484
- Primary Citation of Related Structures:
8S59 - PubMed Abstract:
Glutamine synthetases (GS) are central enzymes essential for the nitrogen metabolism across all domains of life. Consequently, they have been extensively studied for more than half a century. Based on the ATP-dependent ammonium assimilation generating glutamine, GS expression and activity are strictly regulated in all organisms. In the methanogenic archaeon Methanosarcina mazei , it has been shown that the metabolite 2-oxoglutarate (2-OG) directly induces the GS activity. Besides, modulation of the activity by interaction with small proteins (GlnK 1 and sP26) has been reported. Here, we show that the strong activation of M. mazei GS (GlnA 1 ) by 2-OG is based on the 2-OG dependent dodecamer assembly of GlnA 1 by using mass photometry (MP) and single particle cryo-electron microscopy (cryo-EM) analysis of purified strep-tagged GlnA 1 . The dodecamer assembly from dimers occurred without any detectable intermediate oligomeric state and was not affected in the presence of GlnK 1 . The 2.39 Å cryo-EM structure of the dodecameric complex in the presence of 12.5 mM 2-OG demonstrated that 2-OG is binding between two monomers. Thereby, 2-OG appears to induce the dodecameric assembly in a cooperative way. Furthermore, the active site is primed by an allosteric interaction cascade caused by 2-OG-binding towards an adaption of an open active state conformation. In the presence of additional glutamine, strong feedback inhibition of GS activity was observed. Since glutamine dependent disassembly of the dodecamer was excluded by MP, feedback inhibition most likely relies on the binding of glutamine to the catalytic site. Based on our findings, we propose that under nitrogen limitation the induction of M. mazei GS into a catalytically active dodecamer is not affected by GlnK 1 and crucially depends on the presence of 2-OG.
Organizational Affiliation:
Institute for General Microbiology, Christian Albrechts University, Kiel, Germany.