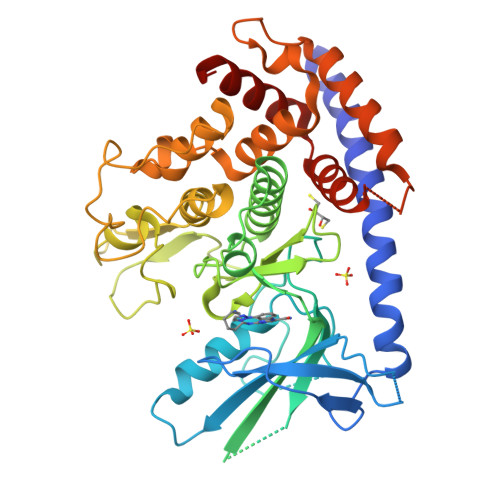
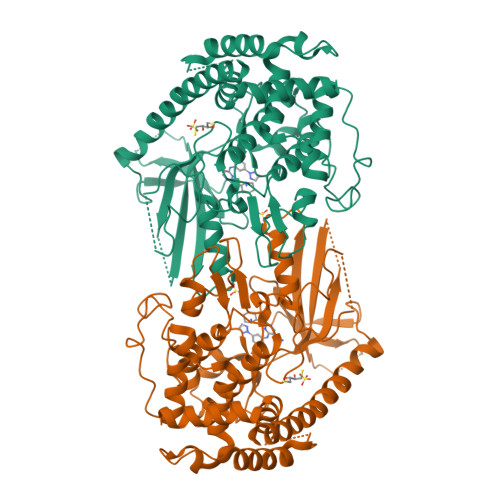
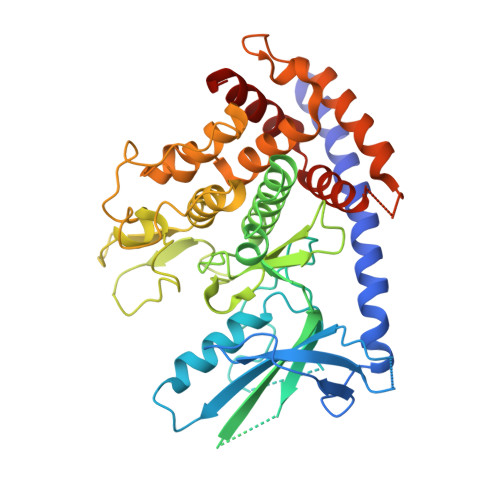
Identification and structural characterization of small molecule inhibitors of PINK1.
Rasool, S., Shomali, T., Truong, L., Croteau, N., Veyron, S., Bustillos, B.A., Springer, W., Fiesel, F.C., Trempe, J.F.(2024) Sci Rep 14: 7739-7739
- PubMed: 38565869
- DOI: https://doi.org/10.1038/s41598-024-58285-3
- Primary Citation of Related Structures:
8UCT, 8UDC - PubMed Abstract:
Mutations in PINK1 and Parkin cause early-onset Parkinson's Disease (PD). PINK1 is a kinase which functions as a mitochondrial damage sensor and initiates mitochondrial quality control by accumulating on the damaged organelle. There, it phosphorylates ubiquitin, which in turn recruits and activates Parkin, an E3 ubiquitin ligase. Ubiquitylation of mitochondrial proteins leads to the autophagic degradation of the damaged organelle. Pharmacological modulation of PINK1 constitutes an appealing avenue to study its physiological function and develop therapeutics. In this study, we used a thermal shift assay with insect PINK1 to identify small molecules that inhibit ATP hydrolysis and ubiquitin phosphorylation. PRT062607, an SYK inhibitor, is the most potent inhibitor in our screen and inhibits both insect and human PINK1, with an IC 50 in the 0.5-3 µM range in HeLa cells and dopaminergic neurons. The crystal structures of insect PINK1 bound to PRT062607 or CYC116 reveal how the compounds interact with the ATP-binding pocket. PRT062607 notably engages with the catalytic aspartate and causes a destabilization of insert-2 at the autophosphorylation dimer interface. While PRT062607 is not selective for PINK1, it provides a scaffold for the development of more selective and potent inhibitors of PINK1 that could be used as chemical probes.
Organizational Affiliation:
Department of Pharmacology & Therapeutics, Centre de Recherche en Biologie Structurale, and Structural Genomics Consortium, McGill University, 3655 Prom Sir William Osler, Montréal, QC, H3G 1Y6, Canada.