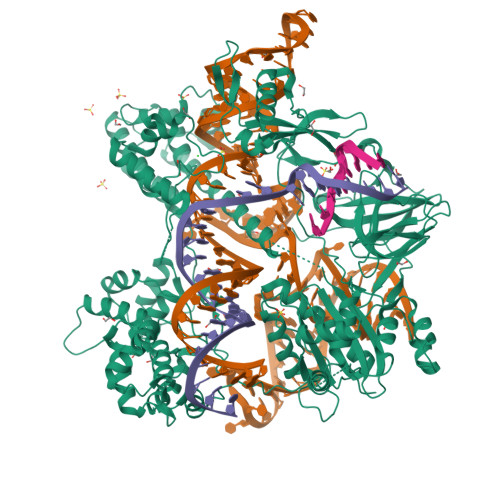
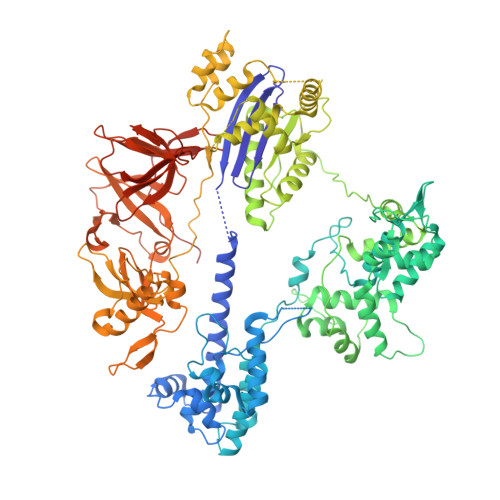
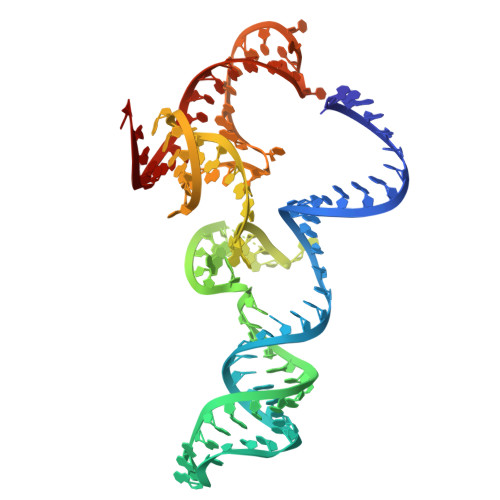
Structure and engineering of Brevibacillus laterosporus Cas9.
Nakane, T., Nakagawa, R., Ishiguro, S., Okazaki, S., Mori, H., Shuto, Y., Yamashita, K., Yachie, N., Nishimasu, H., Nureki, O.(2024) Commun Biol 7: 803-803
- PubMed: 38961195
- DOI: https://doi.org/10.1038/s42003-024-06422-z
- Primary Citation of Related Structures:
8X5V - PubMed Abstract:
The RNA-guided DNA endonuclease Cas9 cleaves double-stranded DNA targets complementary to an RNA guide, and is widely used as a powerful genome-editing tool. Here, we report the crystal structure of Brevibacillus laterosporus Cas9 (BlCas9, also known as BlatCas9), in complex with a guide RNA and its target DNA at 2.4-Å resolution. The structure reveals that the BlCas9 guide RNA adopts an unexpected architecture containing a triple-helix, which is specifically recognized by BlCas9, and that BlCas9 recognizes a unique N 4 CNDN protospacer adjacent motif through base-specific interactions on both the target and non-target DNA strands. Based on the structure, we rationally engineered a BlCas9 variant that exhibits enhanced genome- and base-editing activities with an expanded target scope in human cells. This approach may further improve the performance of the enhanced BlCas9 variant to generate useful genome-editing tools that require only a single C PAM nucleotide and can be packaged into a single AAV vector for in vivo gene therapy.
Organizational Affiliation:
Department of Biological Sciences, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan.