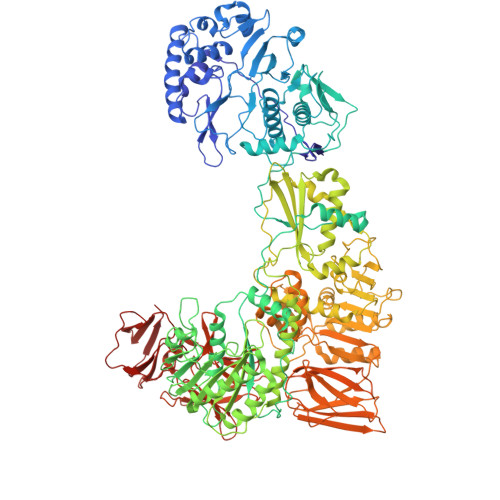
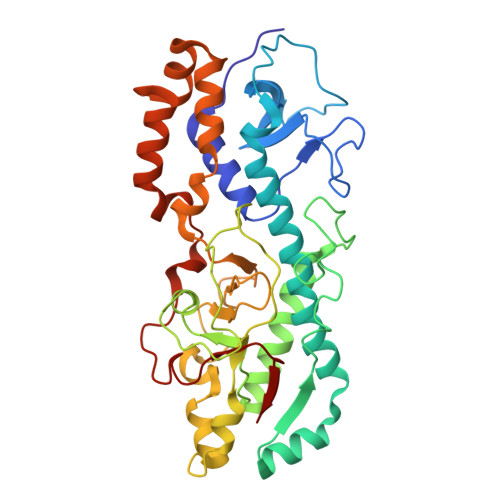
Molecular sociology of virus-induced cellular condensates supporting reovirus assembly and replication.
Liu, X., Xia, X., Martynowycz, M.W., Gonen, T., Zhou, Z.H.(2024) Nat Commun 15: 10638-10638
- PubMed: 39639006
- DOI: https://doi.org/10.1038/s41467-024-54968-7
- Primary Citation of Related Structures:
9CYT, 9CYX, 9CYY - PubMed Abstract:
Virus-induced cellular condensates, or viral factories, are poorly understood high-density phases where replication of many viruses occurs. Here, by cryogenic electron tomography (cryoET) of focused ion beam (FIB) milling-produced lamellae of mammalian reovirus (MRV)-infected cells, we visualized the molecular organization and interplay (i.e., "molecular sociology") of host and virus in 3D at two time points post-infection, enabling a detailed description of these condensates and a mechanistic understanding of MRV replication within them. Expanding over time, the condensate fashions host ribosomes at its periphery, and host microtubules, lipid membranes, and viral molecules in its interior, forming a 3D architecture that supports the dynamic processes of viral genome replication and capsid assembly. A total of six MRV assembly intermediates are identified inside the condensate: star core, empty and genome-containing cores, empty and full virions, and outer shell particle. Except for star core, these intermediates are visualized at atomic resolution by cryogenic electron microscopy (cryoEM) of cellular extracts. The temporal sequence and spatial rearrangement among these viral intermediates choreograph the viral life cycle within the condensates. Together, the molecular sociology of MRV-induced cellular condensate highlights the functional advantage of transient enrichment of molecules at the right location and time for viral replication.
Organizational Affiliation:
Department of Microbiology, Immunology and Molecular Genetics, University of California, Los Angeles, CA, USA.