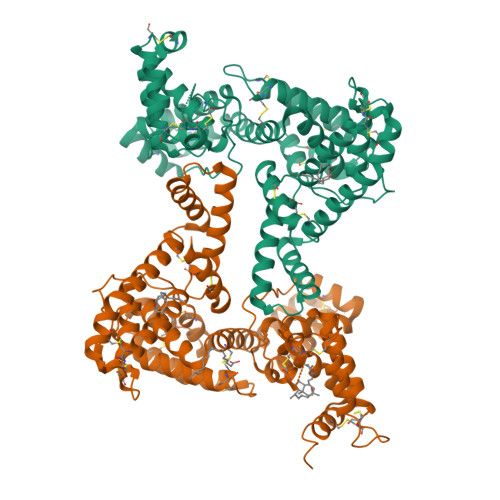
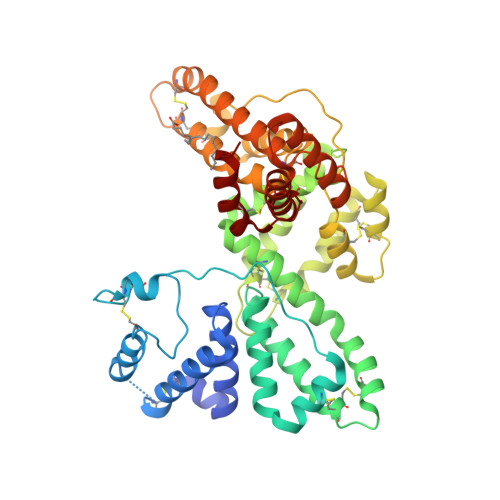
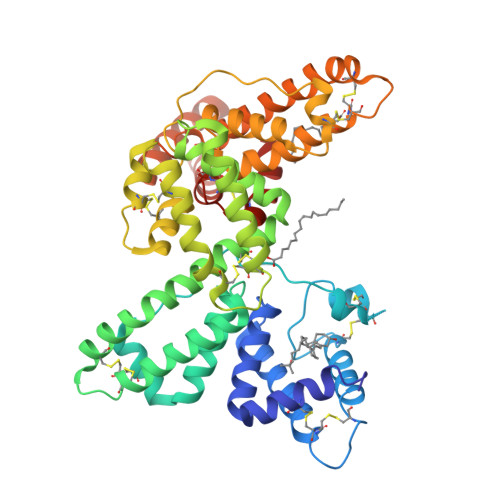
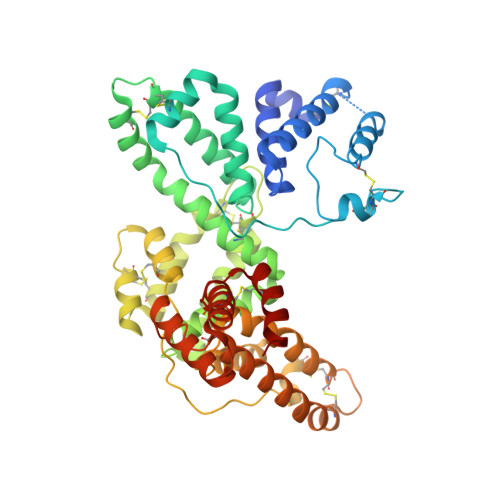
A structural basis for the unique binding features of the human vitamin D-binding protein.
Verboven, C., Rabijns, A., De Maeyer, M., Van Baelen, H., Bouillon, R., De Ranter, C.(2002) Nat Struct Biol 9: 131-136
- PubMed: 11799400
- DOI: https://doi.org/10.1038/nsb754
- Primary Citation of Related Structures:
1J78, 1J7E - PubMed Abstract:
The human serum vitamin D-binding protein (DBP) has many physiologically important functions, ranging from transporting vitamin D3 metabolites, binding and sequestering globular actin and binding fatty acids to functioning in the immune system. Here we report the 2.3 A crystal structure of DBP in complex with 25-hydroxyvitamin D3, a vitamin D3 metabolite, which reveals the vitamin D-binding site in the N-terminal part of domain I. To more explicitly explore this, we also studied the structure of DBP in complex with a vitamin D3 analog. Comparisons with the structure of human serum albumin, another family member, reveal a similar topology but also significant differences in overall, as well as local, folding. These observed structural differences explain the unique vitamin D3-binding property of DBP.
Organizational Affiliation:
Laboratorium voor Analytische Chemie en Medicinale Fysicochemie, Faculteit Farmaceutische Wetenschappen, Katholieke Universiteit Leuven, E. Van Evenstraat 4, B-3000 Leuven, Belgium.