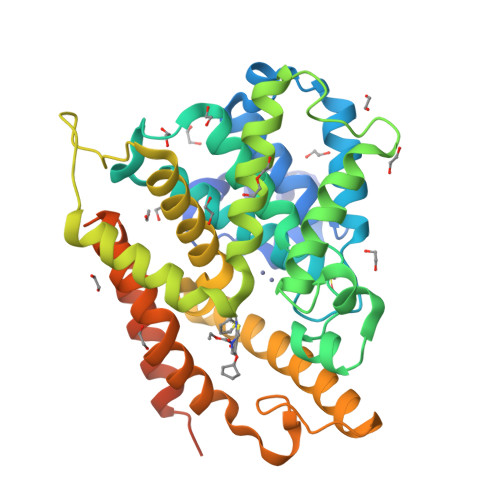
Thiophene inhibitors of PDE4: Crystal structures show a second binding mode at the catalytic domain of PDE4D2.
Nankervis, J.L., Feil, S.C., Hancock, N.C., Zheng, Z., Ng, H.L., Morton, C.J., Holien, J.K., Ho, P.W., Frazzetto, M.M., Jennings, I.G., Manallack, D.T., Martin, T.J., Thompson, P.E., Parker, M.W.(2011) Bioorg Med Chem Lett 21: 7089-7093
- PubMed: 22030030
- DOI: https://doi.org/10.1016/j.bmcl.2011.09.109
- Primary Citation of Related Structures:
3SL3, 3SL4, 3SL5, 3SL6, 3SL8 - PubMed Abstract:
PDE4 inhibitors have been identified as therapeutic targets for a variety of conditions, particularly inflammatory diseases. We have serendipitously identified a novel class of phosphodiesterase 4 (PDE4) inhibitor during a study to discover antagonists of the parathyroid hormone receptor. X-ray crystallographic studies of PDE4D2 complexed to four potent inhibitors reveal the atomic details of how they inhibit the enzyme and a notable contrast to another recently reported thiophene-based inhibitor.
Organizational Affiliation:
Medicinal Chemistry & Drug Action, Monash Institute of Pharmaceutical Sciences, Monash University (Parkville campus), Parkville, Victoria, Australia.