Chemical Proteomics Defines the Mammalian N- Myristoylated Proteome in Live Cells Global Profiling of Co- and Post-Translationally N-Myristoylated Proteomes in Human Cells.
Thinon, E., Serwa, R.A., Broncel, M., Brannigan, J.A., Brassat, U., Wright, M.H., Heal, W.P., Wilkinson, A.J., Mann, D.J., Tate, E.W.(2014) Nat Commun 5: 4919
- PubMed: 25255805
- DOI: https://doi.org/10.1038/ncomms5919
- Primary Citation of Related Structures:
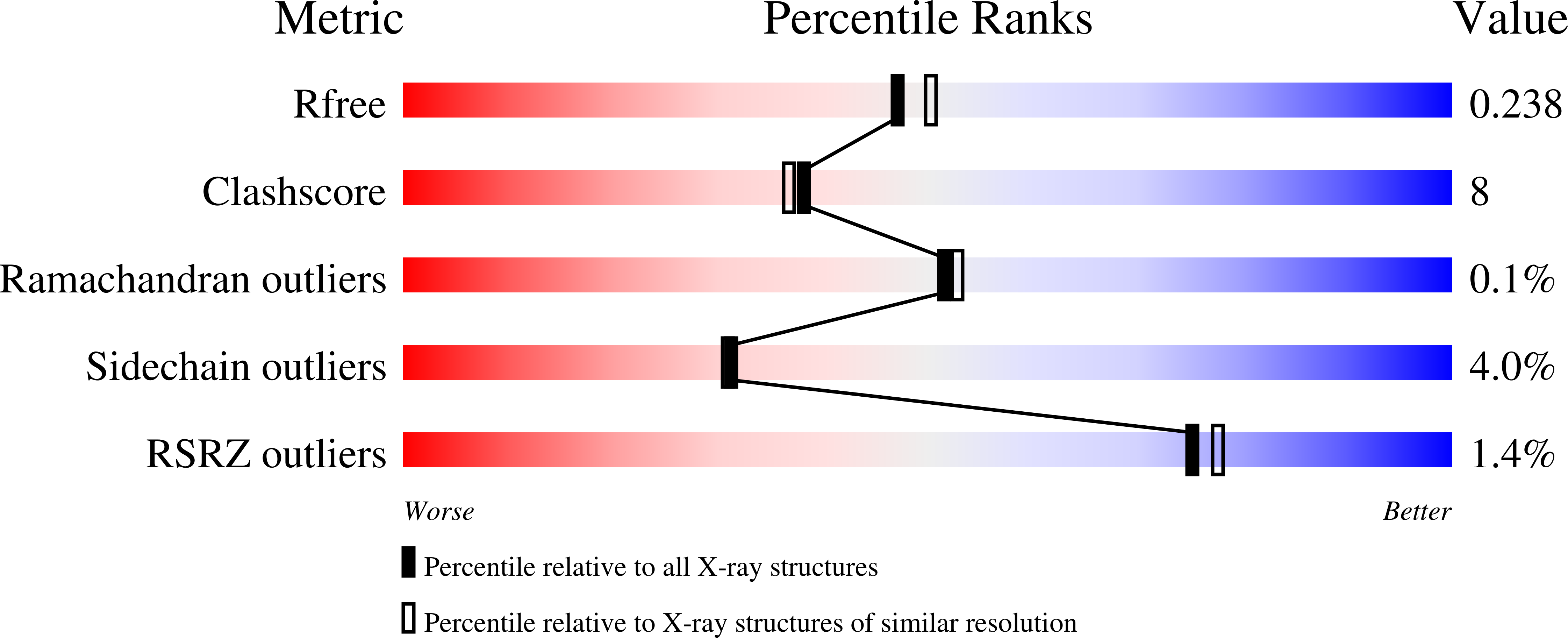
4C2X, 4C2Y, 4C2Z - PubMed Abstract:
Protein N-myristoylation is a ubiquitous co- and post-translational modification that has been implicated in the development and progression of a range of human diseases. Here, we report the global N-myristoylated proteome in human cells determined using quantitative chemical proteomics combined with potent and specific human N-myristoyltransferase (NMT) inhibition. Global quantification of N-myristoylation during normal growth or apoptosis allowed the identification of >100 N-myristoylated proteins, >95% of which are identified for the first time at endogenous levels. Furthermore, quantitative dose response for inhibition of N-myristoylation is determined for >70 substrates simultaneously across the proteome. Small-molecule inhibition through a conserved substrate-binding pocket is also demonstrated by solving the crystal structures of inhibitor-bound NMT1 and NMT2. The presented data substantially expand the known repertoire of co- and post-translational N-myristoylation in addition to validating tools for the pharmacological inhibition of NMT in living cells.
Organizational Affiliation:
1] Department of Chemistry, Imperial College London, Exhibition Road, London SW7 2AZ, UK [2] Department of Life Sciences, Imperial College London, Exhibition Road, London SW7 2AZ, UK.