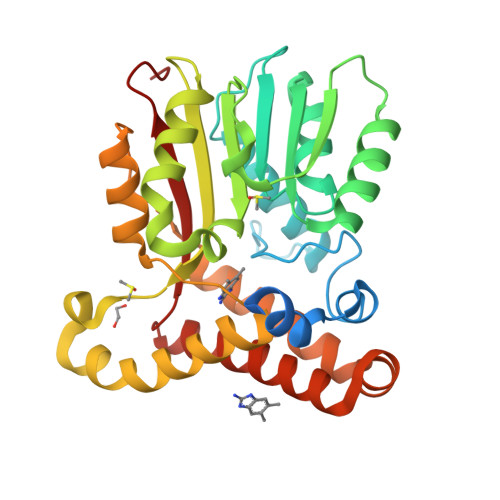
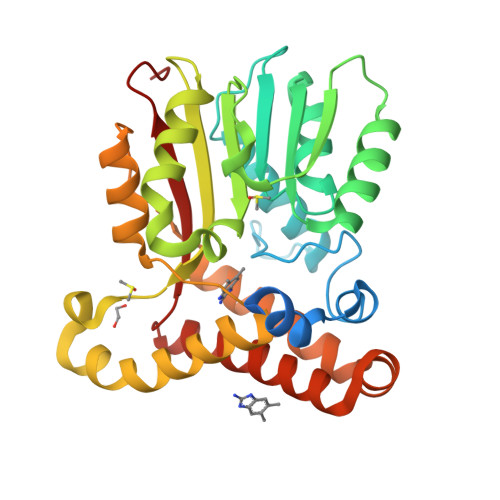
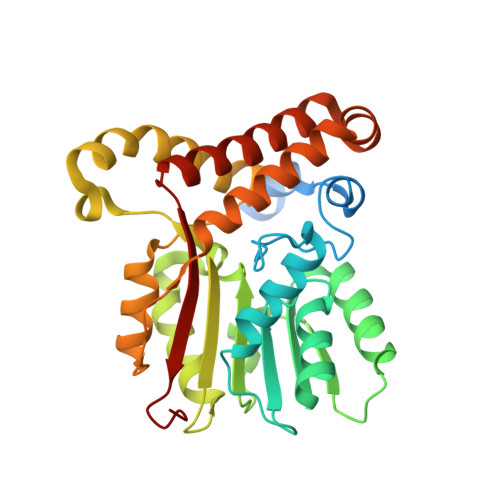
Fragment-Based Ligand Discovery Applied to the Mycolic Acid Methyltransferase Hma (MmaA4) from Mycobacterium tuberculosis : A Crystallographic and Molecular Modelling Study.
Galy, R., Ballereau, S., Genisson, Y., Mourey, L., Plaquevent, J.C., Maveyraud, L.(2021) Pharmaceuticals (Basel) 14
- PubMed: 34959681
- DOI: https://doi.org/10.3390/ph14121282
- Primary Citation of Related Structures:
7Q2B, 7Q2C, 7Q2D, 7Q2E, 7Q2F, 7Q2G, 7Q2H - PubMed Abstract:
The mycolic acid biosynthetic pathway represents a promising source of pharmacological targets in the fight against tuberculosis. In Mycobacterium tuberculosis , mycolic acids are subject to specific chemical modifications introduced by a set of eight S-adenosylmethionine dependent methyltransferases. Among these, Hma (MmaA4) is responsible for the introduction of oxygenated modifications. Crystallographic screening of a library of fragments allowed the identification of seven ligands of Hma. Two mutually exclusive binding modes were identified, depending on the conformation of residues 147-154. These residues are disordered in apo -Hma but fold upon binding of the S-adenosylmethionine (SAM) cofactor as well as of analogues, resulting in the formation of the short η1-helix. One of the observed conformations would be incompatible with the presence of the cofactor, suggesting that allosteric inhibitors could be designed against Hma. Chimeric compounds were designed by fusing some of the bound fragments, and the relative binding affinities of initial fragments and evolved compounds were investigated using molecular dynamics simulation and generalised Born and Poisson-Boltzmann calculations coupled to the surface area continuum solvation method. Molecular dynamics simulations were also performed on apo -Hma to assess the structural plasticity of the unliganded protein. Our results indicate a significant improvement in the binding properties of the designed compounds, suggesting that they could be further optimised to inhibit Hma activity.
Organizational Affiliation:
Institut de Pharmacologie et de Biologie Structurale, Université Toulouse III-Paul Sabatier, Centre National de la Recherche Scientifique, 31077 Toulouse, France.