Assembly of the Xrn2/Rat1-Rai1-Rtt103 termination complexes in mesophilic and thermophilic organisms
Dikunova, A., Noskova, N., Overbeck, J.H., Polak, M., Stelzig, D., Zapletal, D., Kubicek, K., Novacek, J., Sprangers, R., Stefl, R.(2024) Structure
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
(2024) Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 5'-3' exoribonuclease 2 | A [auth B] | 1,006 | Saccharomyces cerevisiae | Mutation(s): 0 Gene Names: RAT1 EC: 3.1.13 | 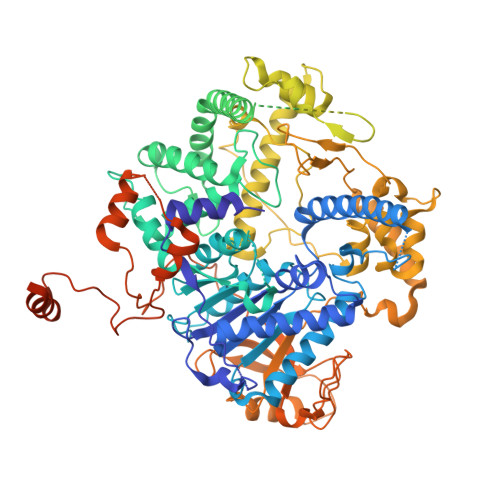 |
UniProt | |||||
Find proteins for Q02792 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore Q02792 Go to UniProtKB: Q02792 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q02792 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Regulator of Ty1 transposition protein 103 | B [auth D] | 22 | Saccharomyces cerevisiae | Mutation(s): 0 |  |
UniProt | |||||
Find proteins for Q05543 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore Q05543 Go to UniProtKB: Q05543 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q05543 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Decapping nuclease RAI1 | C [auth A] | 387 | Saccharomyces cerevisiae | Mutation(s): 0 Gene Names: RAI1, YGL246C, NRE387 EC: 3.6.1 |  |
UniProt | |||||
Find proteins for P53063 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P53063 Go to UniProtKB: P53063 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P53063 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MG (Subject of Investigation/LOI) Query on MG | D [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC | 4.4.1 |
| MODEL REFINEMENT | Coot | 0.9.8.7 |
| MODEL REFINEMENT | ISOLDE |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Czech Science Foundation | Czech Republic | -- |